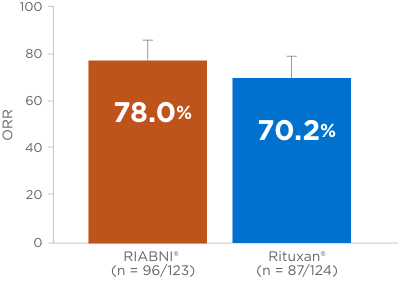
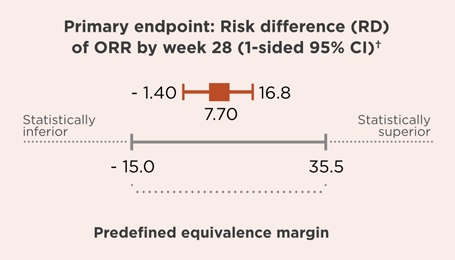
RIABNI® efficacy proven similar to Rituxan® with no clinically meaningful differences
JASMINE: Randomized, double-blind international study that demonstrated clinical similarity of RIABNI® to Rituxan® (6 infusions of 375 mg/m2 at weeks 1-4, 12, 20) in patients* with CD20-positive previously-untreated follicular lymphoma (FL).1,3,6
OVERALL RESPONSE RATE (ORR) RIABNI® VS RITUXAN® (primary endpoint)1,3
- ORR = complete response + unconfirmed complete response + partial response.
- Secondary endpoints: RD of ORR at week 12, percentage of patients with complete depletion of CD19 cell count and total levels of IgE and IgM (baseline to day 8), immunogenicity of RIABNI® vs Rituxan®, progression-free survival (PFS) and overall survival (OS).1,3,6
At the end of the study, all patients were alive and median PFS was not reached (both treatment arms)3
- *Patients (N = 250) with confirmed B-cell FL grade 1, 2, or 3a; low tumor burden, ≥ 18 years of age were enrolled from 150 sites in Europe, North America, Latin America, and Asia Pacific.1,3
-
†Central review.